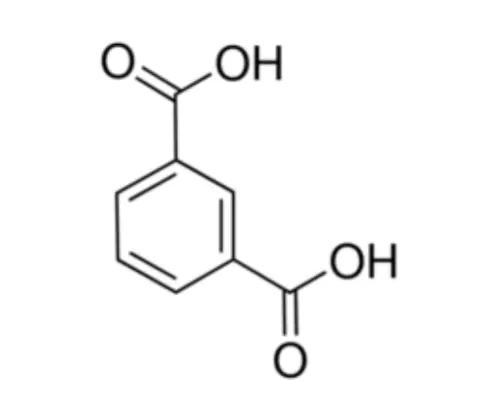
1.Improved thermal properties
Isophthalic acid a rigid benzene ring structure, which can restrict the movement of its molecular chains. When added to polyester resins, it can strengthen the rigidity of the molecular chains, increasing the glass transition temperature and melting point of the resin. This allows the material to maintain stable performance at higher temperatures, especially suitable for high-temperature environments, such as cars and appliance casings.
2.Enhanced mechanical properties
Polyester resins composed of isophthalic acid not only have good strength but also excellent toughness The structure of isophthalic acid can promote flexible connections between molecular chains. This allows the resin to efficiently absorb energy through molecular chain deformation when facing external force, thus reducing the of brittle fracture.
3.Improved environmental resistance
The addition of isophthalic acid can enhance the ability of polyester resins to resist ultraviolet rays, effectively suppress the molecular chain breakage and degradation process caused by ultraviolet rays, thus ensuring that products can maintain long-term use without discoloration, hardening, and other aging issues in outdoor environment. Moreover, in the polyester molecular chain of isophthalic acid, the ester bond is protected by the steric hindrance effect and is not easily dissolved by, providing significant water resistance for isophthalic acid-type polyester resins, so they can maintain stable performance in a humid environment